The first (1) includes 213 patients with a PI-RADS score lesion >2 on MRI. The reference diagnostic test is the combination of 2 to 4 targeted biopsies and 24 systematic transperineal biopsies. The rate of detection of significant cancer (sCa, Gleason score >3+3) is compared to that of nine targeted biopsies, within the tumor and its vicinity. sCa could be detected in 99% at a lesion and a patient level. This rate was higher than that of conventional targeted biopsies (87%). Systematic biopsies only detect non-significant cancers. The authors therefore propose to suppress them.
The second study (2) combines the use of micro-ultrasound (microUS, Exact Imaging, Canada) and MRI to guide transrectal biopsy of suspicious lesions detected by both examinations. Biopsies directed into the MRI lesions were performed using the fusion system of the device (Fusion Vu). Lesions detected by microUS were biopsied under visual control. The study included 159 patients. In 58% of the patients, the Gleason score was the same with both types of guidance. In 26% of patients, it was higher with microUS guidance and in 16% of patients with fusion guidance in the MRI target. In three patients without MRI or microUS abnormality outside the index lesion, three significant cancers (3/159, 1.9%), with a non-reported percent of Gleason grade 4 pattern, were detected. The authors therefore consider it possible to discard systematic biopsies by combining microUS and MRI to guide targeted biopsies.
The results of these studies support the idea that the majority of positive systematic biopsies are actually sampled within or near the target area, detected by MRI and/or by microUS. It therefore makes sense to saturate the target and its vicinity rather than perform systematic biopsies. This strategy relies on a precise localization of the target, and the ideal imaging modality should consistently see the lesion during biopsy.
Direct MRI guidance meet the conditions, because the same examination is used to localize and guide the biopsy. The current limitations are the limited availability of interventional MRI in most countries, partly related to the cost of MRI time and the dissuasive cost of disposable MRI compatible material.
Conventional transrectal ultrasound (TRUS) can be used to localize lesions originating in the peripheral zone, if TRUS is used as a second-look examination, i.e. if the location of the lesion is known by MRI and if the TRUS equipment has very good spatial resolution which is that of most commercially available diagnostic US scanners. The use of navigation image fusion systems helps to localize the lesion on TRUS by overlaying the MRI lesion during TRUS (3). This is made easier if a biplane US probe is used, which matches MRI and TRUS more accurately than with an end-fire TRUS probe, but requires a transperineal approach for the biopsy. Once the overlay has been completed and the lesion becomes visible on TRUS, the biopsy is simple and easy to perform. Operators have to be familiar with prostate MRI and TRUS to be eligible for this image-guided procedure. If these conditions are met, PI-RADS 4 and 5 lesions originating in the peripheral zone (PZ) can be detected in about 80% of cases (3). The limitations are barely visible posterior cancers on ultrasound and anterior cancers that are only anecdotally visible on conventional ultrasound.
Elastic image fusion systems (Artemis, Koelis, Smart Target) can also be used. They do not search to see the lesion, because the technology is based on an elastic (deformable) image fusion of MRI and TRUS volumes. The target is the result of an image registration and is displayed as a tag on the TRUS screen to guide the biopsy. Because the quality of TRUS does not matter, the limitation is the risk or missing the target due to image registration errors. To circumvent this limitation, it has been suggested to combine registered with cognitive biopsies during the same session to saturate the presumed area of the MRI lesion (4).
MicroUS guidance (Exact Imaging, Canada) is a new imaging modality showing a definite improvement in ultrasound imaging, as reported in the second study quoted above (2). MicroUS operates with a very high frequency TRUS probe (29MHZ). We used it as a second look examination and showed that PZ and lower third TZ cancers could be detected in 100% of cases, without image fusion in the vast majority of cases, once their location was known on MRI (5). MicroUS could also detect PZ small significant tumors, satellite to the index lesion and not visible on MRI, in about 20% of cases (5). The recent improvement in the depth of exploration from 5 to 6 cm further improves the detection of TZ lesions developed in the middle third of the prostate (Figure 1) and larger volume prostates.
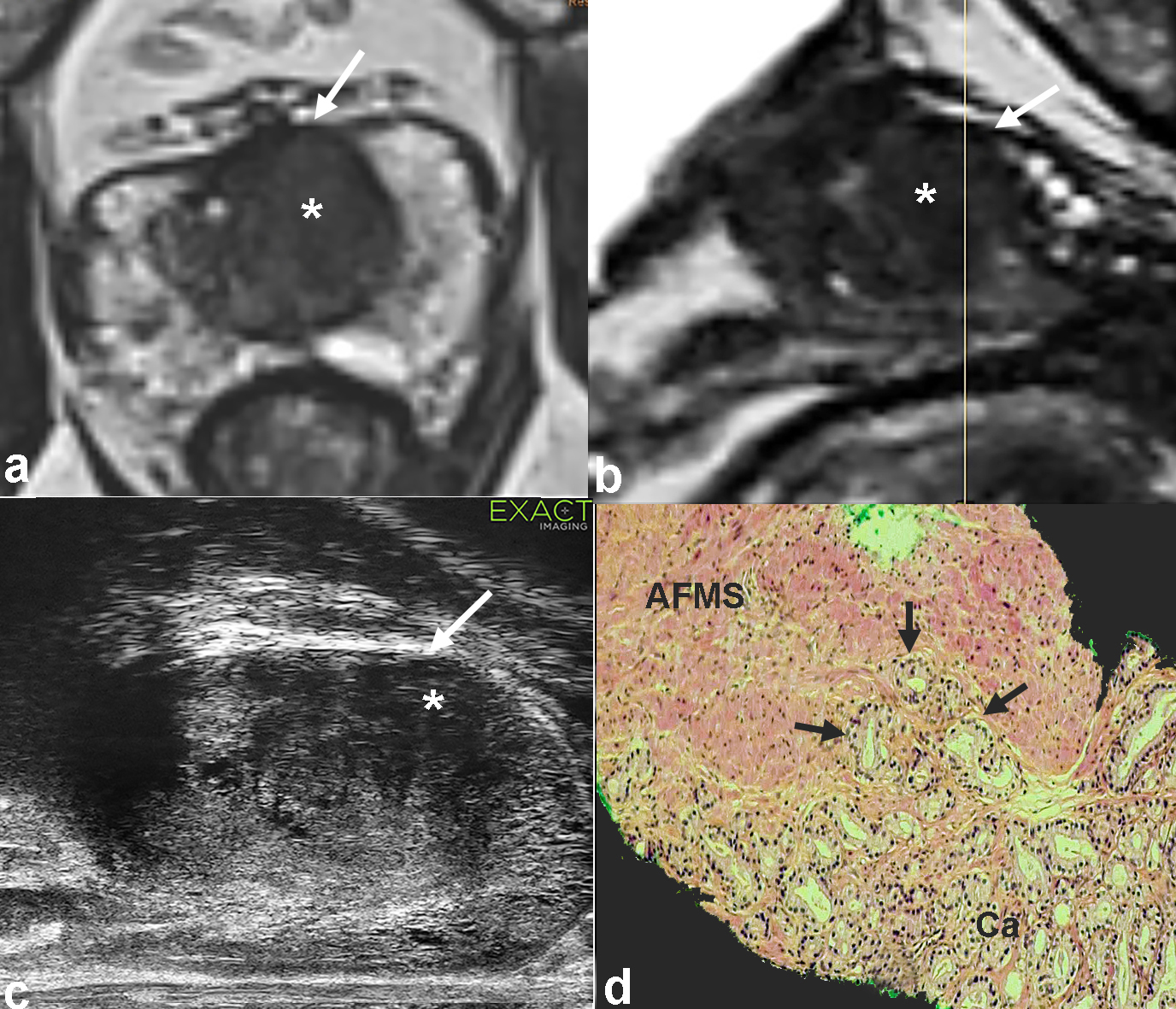
Figure 1 : 67 y/o man. PSA 6 ng/ml. Axial and sagittal T2 weighted images of the multiparametric MRI show a large volume tumor in the left TZ (*, a-b). On microUS, the lesion is well visible (*, c). Note the anterior bulging of the lesion (white arrow, a-c), suggesting that the anterior fibromuscular stroma (SFMA) is infiltrated with a possible extraprostatic extension. The transperineal biopsy shows a Gleason 3+4 carcinoma (30% Grade 4 component), with tumors cells (d, arrows) infiltrating the anterior fibromuscular stroma.
The combination of MRI and microUS guidance makes it possible to concentrate targeted biopsies in the lesion and its close vicinity, as suggested in the first study quoted above (1). It represents the best way to hit the target consistenly while taking into account the heterogeneity of medium and large volume cancers in which the geographic distribution of Gleason 4 grade foci within the tumor is unpredictable. By spreading the samples across and around the entire lesion, the tumor highest detection rate with the highest Gleason score can be achieved more accurately, as shown in previous studies (6, 7).
Except if specific treatment options can be anticipated from the patient profile, it may be time to move away from conventional systematic biopsies.
References
1. Tschirdewahn S, Wiesenfarth M, Bonekamp D, Pullen L, Reis H, Panic A, et al. Detection of Significant Prostate Cancer Using Target Saturation in Transperineal Magnetic Resonance Imaging/Transrectal Ultrasonography-fusion Biopsy. Eur Urol Focus. 2020.
2. Wiemer L, Hollenbach M, Heckmann R, Kittner B, Plage H, Reimann M, et al. Evolution of Targeted Prostate Biopsy by Adding Micro-Ultrasound to the Magnetic Resonance Imaging Pathway. Eur Urol Focus. 2020.
3. van de Ven WJ, Venderink W, Sedelaar JP, Veltman J, Barentsz JO, Futterer JJ, et al. MR-targeted TRUS prostate biopsy using local reference augmentation: initial experience. Int Urol Nephrol. 2016;48(7):1037-45.
4. Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, et al. The SmartTarget Biopsy Trial: A Prospective, Within-person Randomised, Blinded Trial Comparing the Accuracy of Visual-registration and Magnetic Resonance Imaging/Ultrasound Image-fusion Targeted Biopsies for Prostate Cancer Risk Stratification. Eur Urol. 2018;75(5):733-40.
5. Cornud F, Lefevre A, Flam T, Dumonceau O, Galiano M, Soyer P, et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: initial results of a single-center study. Eur Radiol. 2020.
6. Hansen NL, Barrett T, Lloyd T, Warren A, Samel C, Bratt O, et al. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 2020;125(2):260-9.
7. Zhang M, Milot L, Khalvati F, Sugar L, Downes M, Baig SM, et al. Value of Increasing Biopsy Cores per Target with Cognitive MRI-targeted Transrectal US Prostate Biopsy. Radiology. 2019;291(1):83-9.